Rotational spectroscopy uses low-frequency light (microwaves/millimeter waves) to probe the rotational energy levels of polar molecules in the gas phase. Because a molecule’s rotational energy levels are extremely sensitive to its overall structure, a rotational spectrum effectively serves as a unique fingerprint. For this reason, the vast majority of the molecular identifications in space have been made through rotational spectroscopy using large radiotelescopes such as the Green Bank Telescope and the Atacama Large Millimeter Array. In addition to its importance for astrochemistry, rotational spectroscopy can measure precise molecular structures and can probe various types of intra- and intermolecular dynamics, such as internal rotation and tunneling motions.
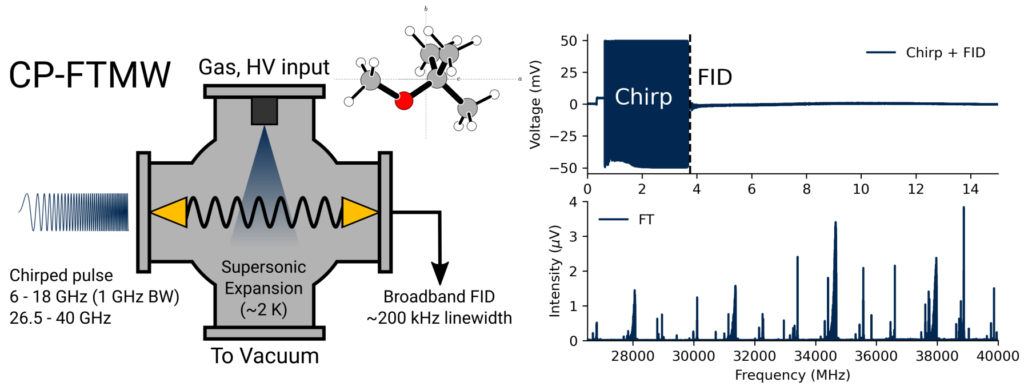
In the Crabtree lab, we use a technique called chirped-pulse Fourier transform microwave (CP-FTMW) spectroscopy. Our sample of interest is entrained in a high-pressure gas (e.g., argon or neon) and introduced into a vacuum chamber through a pulsed valve, generating a supersonic expansion. Inside the chamber, the molecules are irradiated by a high-power “chirp” of microwave radiation which lasts a couple of microseconds. In our instrument, this chirp spans a frequency range of 26.5-40 GHz. In a close analogy to nuclear magnetic resonance spectroscopy, polar molecules with rotational transitions in this frequency range are coherently excited and subsequently generate a free induction decay (FID) which contains their characteristic transition frequencies. The Fourier transform of that FID gives our rotational spectrum.
Our rotational spectroscopy research has two main themes.
1.) Discovery of novel reactive and/or exotic molecules
The extreme conditions present in astrophysical environments such as interstellar clouds and star-forming regions generate a host of exotic molecules, such as radicals and ions. Such species are quite reactive and are challenging to isolate under laboratory conditions. Our CP-FTMW spectrometer is equipped with a high-voltage electrical discharge which generates a plasma. In the plasma, electrons can be blasted off of molecules, bonds can break, and molecular fragments can recombine in new ways to create new species. The subsequent supersonic expansion “freezes” and isolates these newly-formed species, allowing them to be detected by their rotational spectra.
However, many radicals have not been previously studied in the laboratory, so we rely on high-level ab initio quantum chemical calculations to predict the structures and spectroscopic patterns that radicals and other exotic species exhibit. Once we positively identify a new radical in the laboratory, we are able to generate an accurate catalog of its rotational transition frequencies which can subsequently be searched for in space using radiotelescopes.
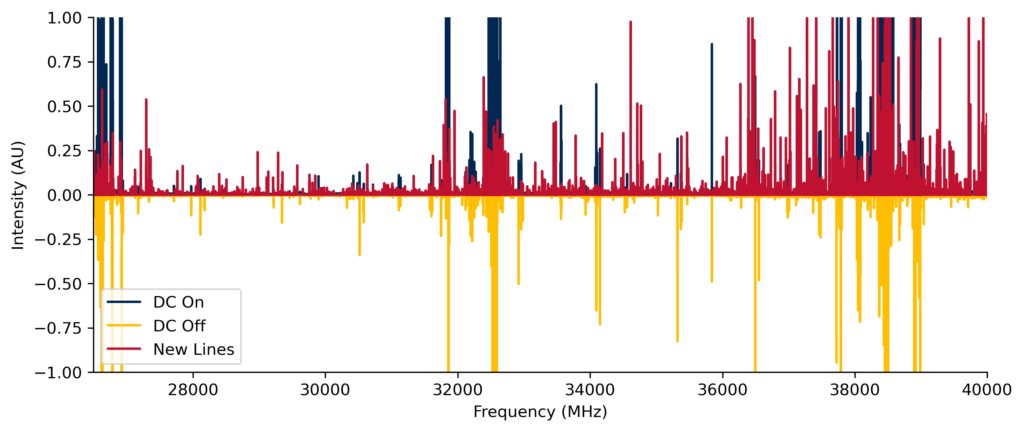
In this project, we are currently exploring nitrogen-containing radicals that may be related to nitrogen heterocycles (N-heterocycles), which are essential components of nucleobases and other biologically relevant molecules. All of the DNA nucleobases and many other related structures have been detected on meteorites with extraterrestrial origins, yet no N-heterocycle has been detected in space to date. We are exploring radicals which may be reactants leading to the formation of N-heterocycles or which may be signatures of their decomposition. Our early work in this area led to the first laboratory detection of the β-cyanovinyl radical (HC=CHCN), which may be an astronomical precursor of pyridine (c-C5H5N). We are now searching for the pyrrolyl (c-C4H4N) and pyridyl (c-C5H4N) radicals using CP-FTMW spectroscopy. To do this, we create electrical discharges of gas mixtures containing samples such as vinyl cyanide (C2H3CN), pyrrole (c-C4H5N), pyridine, and/or other related species. The rotational spectra of the plasmas we generate contain thousands of spectroscopic transitions that may arise from many tens or hundreds of unique molecules, many of which may not have been previously reported.
2.) Measuring intramolecular dynamics of N-containing ring molecules
In collaboration with Alicia Hernandez-Castillo (Harvey Mudd College), we have implemented a heated reservoir into our pulsed gas source which allows us to measure rotational spectra of samples which are solid at room temperature. Using this source, we have investigated the rotational spectra of various succinimide and pyrrolidone molecules, which are saturated analogs of some of the classes of N-heterocycles identified on meteorites. Some molecules in this family show pseudorotation, a type of tunneling motion related to ring puckering which leads to additional splittings in the rotational spectrum. From the magnitudes of the splittings, we can extract information about the barrier to pseudorotation on top of the normal structural information afforded by rotational spectroscopy.
This project also relies on the interplay of ab initio quantum mechanical calculations and experiments. For each molecule we study, we use various levels of electronic structure theory to predict the structures and relative energies of its conformers, and identify potential pseudorotation and other large-amplitude motion pathways and barriers. The results of the calculations give us initial predictions for the rotational spectra, which we then use to assist with the interpretation of the experimental spectra we measure by CP-FTMW spectroscopy.

